Properties of Liquid
Properties of Liquid – Substance is something that has mass and can occupy a space. In every object consists of a substance or material. There are many examples of objects which are forms of the substance itself. Like a stone, air, wind, and many more.
Liquid is a substance in the form of fluid, which has the nature of occupying space according to the shape of the container. Liquid substances have properties,shapes and examples are very diverse.
On this occasion YukSinau.co.id will discuss the complete nature of liquid substances, for more details, let us consider the following discussion.
Table of Contents
Properties of Liquid

& ldquo; The Liquid substance has a certain volume but does not have a fixed shape, because the liquid form follows the container it is in”
Liquid is often referred to as fluid or fluid. Zalir is a substance that has particles that move freely and pass through one another. Zalir will follow the form according to the container.
Liquid substances have a molecular arrangement or arrangement of particles that is tenuous or irregular, thus making the liquid difficult to compress.
But these particles have enough energy to overcome the attraction between molecules that are nearby, so that the particles can shift and can pass each other.
And also liquid substances have a fixed volume, but the shape can change according to the container in place. The following are the properties of liquid.
- Liquid presses in all directions.
- Flows from higher to lower.
- Having free particle motion.
- Put the particles closer together.
- Irregular arrangement of particles.
- The surface is always flat.
Characteristics of Liquid Substances
Besides having some of the properties above, liquid substances also have the following characteristics.
| 1 | Particle Motion | Free |
| 2 | Volume | Permanent |
| 3 | Shape | Always changing to follow the container in place |
| 4 | Molecular Layer | Nearby |
| 5 | Arrangement of Particles | Tenuous |
| 6 | Pull Force Between Particles | Weak |
Besides having 6 properties of a liquid also have a difficult feature to compress because a liquid has a finite particle arrangement.
Liquid Expansion Formula
Every substance will surely expand if the temperature is raised, with the exception of water, because if the water is heated from 00C to 40C then the water will shrink. The peculiar nature of water is also called the water anomaly.
In liquids there is only a volume expansion reaction, so that the liquid only gets the following expansion equation.
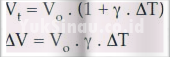
Apart from the above equation the liquid is also formulated as follows.
| ΔV = Vo.ΔT.b |
Information :
ΔV = Volume change on the object
Vo = start volume – start
ΔT = temperature change on the object
b = coefficient
The following is an efficient space for the types of liquid .
| No. | Types of Liquid Substances | Expansion coefficient |
| 1. | Mercury. | 0,0002 |
| 2. | Air. | 0,0004 |
| 3. | Glycerine. | 0,0005 |
| 4. | Paraffin oil. | 0,0009 |
| 5. | alcohol. | 0,0012 |
Water anomaly
Anomalous water is a characteristic of water where the volume of water increases when it is cooled starting from 4 °C. Most substances, if the substance is heated it will expand, but that doesn't apply to water.
Anomaly in water expansion is the nature of water that is not much different from other substances, where the water in a state of expansion so that the volume will increase and not shrink when the temperature decreases from 4 °C ke 0 °C. This results in ice having a density or density smaller than water.
But the opposite, water will shrink causing the volume of water to decrease rather than expand when the temperature is raised from 0 °C ke 4 °C. The change in density occurs because water molecules will form open crystals where the distance will change with temperature.
The result of this water anomaly is, water expansion becomes non-linear, and water is not suitable if used as a thermometer filler. This is clearly different from mercury or mercury, mercury in the form of metal which has a linear expansion and will expand if the temperature is raised.
Ice that has properties that expand when frozen has an impact on the environment. Because frozen ice has a lower density than cold water, therefore ice will float on water.
Another impact is, an increase in the volume of frozen ice will cause tissue damage in living things that exist at freezing temperatures.
Examples of Liquid Substances
There are many examples of liquid substances in everyday life, examples are as follows :
- alcohol
- Air
- Ethanol
- Mercury
- spritus
- Fluid in a chemical laboratory
- Milk
- Honey
- Soy sauce
- Blood
- Sweat
- urine
- Dew
- Intravenous fluids
- and many more
Differences in the Properties of Liquid Substances, Gas and Solid Substances
Each substance must have different properties, in everyday life we know 3 type of substance viz, liquid, solids and gases. Then what is the difference between these three substances.
The following is a table of the differences between the properties of solids, liquid, and gas substances.
| Nature | Liquid substances | Solid substance | Gas substance |
| Shape | Following the shape of the container in his place |
Permanent | Following the container in place |
| Mass Type | Is | Big | Small |
| Volume | Permanent | Permanent | Depending on the place |
Changes in the Form of Liquid Substances
As we know that each substance can change shape, like a liquid becomes dense, gas becomes liquid, dense to liquid and much more. Then what are the changes in the shape of the liquid object?
For more details, let us consider the following explanation of changes in liquid form.
- Yawn, Yawning is a change in liquid into a gas. An example is water that evaporates when boiled.
- Froze up, freezing is the change of liquid into frozen. An example is when we make ice cubes.
- Condense, condensation is the transformation of a gas substance into a liquid. Examples are dew
- Melt / melt, melting is the change of a solid into a liquid. Examples are burning candles.
Examples of Questions About Liquid Substances
A. A Pot is filled with as much water 5 liter, then the pan is heated until the temperature rises by 100°C. What is the volume of water that will spill from the pan?
Answer:
Known : Volume air = 5 liter
Temperature = 100°C
The coefficient of expansion of water = 0.0004 /°C
Asked : volume of water spilled
Answer : Spilled water volume = increase in volume due to heating
ΔV = Vo.ΔT.b
ΔV = 5 x 100 x 0,0004 = 0,2
So the volume of water that spills from the pan as much 0,2 liter.
B. A glass size 100 ml in a lab filled with 90ml alcohol. Then the measuring cup is placed on the fire and heated so that the temperature rises to 120°C. What is the volume of alcohol that will house from the beaker glass?
Answer :
Known : Volume of alcohol in beaker glass 90 ml = 0,09 liter
Temperature = 120°C
Alcohol expansion coefficient = 0.0012 /°C
Asked : Alcohol spilled from the beaker glass
Answer : ΔV = Vo.ΔT.b
ΔV = 0,09 x 120 x 0,0012 = 0.01296/°C.
So the volume of alcohol that is home from the geals beaker is as much as 0.01296 /°C.
Thus our explanation of the "Properties of Liquid Substances" rdquo;, hopefully can be useful as learning material. To find out other physics material visit the article below.
Other Articles :
- Gauss's Law
- Lenz's Law
- Electromagnetic wave
- Black Body Radiation
- Potential Energy Formula
- Unidirectional Electric Current
- Gas Laws
The post Sifat Zat Cair appeared first on YukSinau.co.id.
