Gas Laws
Gas Laws – The state of the gas in the enclosed space can be expressed in terms of temperature, volume, and pressure. Magnitude- that amount is called the state variable. If one variable changes, then other variables can be determined.
It was at the end of the 18th century when scientists realized the relationship between volumes, pressure and temperature from a gas sample can be obtained, which forms the basis for the approach of all gases, they began to develop it.
On this occasion YukSinau.co.id will discuss in full about the gas law in physics, let's consider the following explanation.
Table of Contents
Gas Laws
Gas law is a law that covers gas, in physics there are several gas laws that are often used. Among them is Boyle's Law, Hukum Gay-Lussac, Charles Law, Hukum Boyle-Gay Lussac, and several other ideal gas laws.
Boyle's Law

Boyle's Law was first stated in the year 1662, by a physicist named Robert Boyle. By using a pressure gauge and a variable volume container, the law can be verified in an experimental manner.
Boyle's Law reads "The volume of a gas is inversely proportional to the pressure applied when the temperature is constant”.
Boyle's Law can be written mathematically using the following formula:
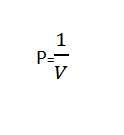
Or
| P.V = Constant |
The formula above applies to gas with different conditions, but at the same temperature, the volume and pressure equation can be written as follows :
| P1. V1 = P2.V2 |
Information:
P1 = State gas pressure 1 (Well)
P2 = State gas pressure 2 (Well)
V1 = Volume of gas state 1 (m3)
V2 = Volume of gas state 2 (m3)
Charles Law

Charles Law, discovered by a physicist named Jacques Charles in year 1787. Charles's law states “The volume of a gas is directly proportional to its absolute temperature when the pressure is constant“.
Charles's law can be written mathematically with the following formula :
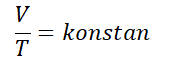
For a gas that is in equilibrium, then one of the variables changes, but the pressure remains, the equation can be written as follows.
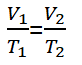
Information:
V1 = Volume of gas state 1 (m3)
V2 = Volume of gas state 2 (m3)
T1 = Gas temperature condition 1 (K)
T2 = Gas temperature condition 2 (K)
Hukum Gay-Lussac

Hukum Gay-Lussac, or also called Amontons law, is a law that was discovered by a physicist named Joseph Louis Gay-Lussac in 1809. Gay-Lussac's Law reads “That at a fixed volume, the gas pressure is proportional to its absolute temperature“.
Mathematically the Gay-Lussac Law can be formulated as follows :
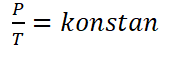
For gases having different equilibrium conditions, but at a fixed volume, Gay-Lussac's law can be formulated as follows.
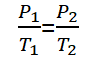
Information :
P1 = State gas pressure 1 (Well)
P2 = State gas pressure 2 (Well)
T1 = Gas temperature condition 1 (K)
T2 = Gas temperature condition 2 (K)
Hukum Boyle-Gay Lussac
The three laws in the bag can be merged as Boyle-Gay Lussac's Law, which is a combination of Boyle's Law, Charles Law, and Gay-Lussac Law.
Lussac's Boyle-Gay Law can be formulated as follows.
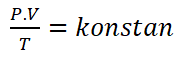
Information :
P = Pressure (Well)
V = Volume (m3)
T = Temperature (K)The combined law has the following important consequences:
Combined gas law has the following consequences.
- When pressure and temperature are constant, then the volume of gas produced will be equal to the number of gas molecules.
- When the temperature and volume remain constant, then the pressure at which gas changes will equal the number of molecules of the gas.
- When the temperature and amount of gas molecules remain constant, then the existing pressure goes backward with volume.
- When the temperature changes the amount of gas molecules remains constant, then the volume and pressure will change.
Avogadro's Law

Avogadro's law is one of the gas laws discovered by a scientist named Amedeo Avogadro in 1811. Avogadro's Law reads “the volume occupied by the ideal gas is the same as the gas molecule in the tar containercall & rdquo;

Information :
V1 = Volume of gas state 1 (m3)
V2 = Volume of gas state 2 (m3)
n = Number of moles of gas
Other Gas Laws
In addition to the Gas Laws above there are several other gas laws such as the following.
- Graham's Law, Graham's Law reads "that the rate at which the diffused gas molecule will contradict the square roots of its density at a constant temperature. This law in combination with Avrogado's law because the same volume will have the same number of molecules, this is the opposite of the root of the molecular weight.
- Dalton's Law, is a law about partial pressure which reads “that a simple gas combined pressure is the amount of partial pressure coming from the individual components of that pressure“.
- Hukum Amagat, is a law that deals with partial volumes, Amagat's law reads ” The volume of a gas mixture or the volume of a container is simply saidh partial volumes derived from these individual components“. n as:
- Henry's Law has a sound “In constant temperature, the amount of gas dissolved in the volume and type of liquid given will be the same as the partial pressure present in the gas in equilibrium in the liquid.”
Thus our discussion of the Gas Laws, hopefully can be useful. To find out about other Physics material can visit the following article.
Other Articles :
- Gauss's Law
- Lenz's Law
- Electromagnetic wave
- Black Body Radiation
- Potential Energy Formula
- Unidirectional Electric Current
- Solid substance
The post Hukum-Hukum tentang Gas appeared first on YukSinau.co.id.
